The Meals and Drug Management spark off a firestorm of dialogue when it permitted a brand new drug, aducanumab, for Alzheimer’s illness by the use of an speeded up approval pathway. This choice left out the advice of the FDA’s exterior advisory panel to reject the drug.
The FDA grants speeded up approvals for medicine to regard severe sicknesses for which there aren’t any recognized, or no less than only a few, therapies. The kind of knowledge used to give a boost to speeded up approvals could be very other from the standard benchmark protection and efficacy knowledge required for approval. As a pharmacist and researcher, I’ve documented a number of causes drug analysis carried out in a laboratory setting differs considerably from what’s in the end noticed in other folks. The problem lies in putting a steadiness between taking the time to verify a remedy works and assembly pressing affected person want.
Contents
The use of a special usual
The FDA created an speeded up approval pathway for medicine treating severe illnesses for which many sufferers really feel a determined want for extra choices. This has integrated remedy for advanced-stage most cancers, more than one sclerosis and HIV, amongst others.
When taking into consideration speeded up approval, the company examines a drug’s efficacy the usage of what’s referred to as a “surrogate endpoint.” Whilst maximum drug trials measure good fortune in line with medical endpoints that resolve whether or not a drug is helping other folks really feel higher or are living longer, like lowering center assaults or strokes, surrogate endpoints measure biomarkers that recommend doable medical receive advantages. Those surrogate endpoints are viable substitutes for laborious medical endpoints as a result of they’re confirmed to be immediately connected to the required medical results. As an example, the medical endpoints of lowering center assaults and strokes may use lowered blood force and low-density lipoprotein (LDL) ldl cholesterol as surrogate endpoints.
Whilst many hypotheses on the proper surrogate endpoints to regard sure illnesses have panned out, a number of others were proven to be off-base or best partly right kind. An ideal instance is homocysteine, an amino acid as soon as regarded as a motive force of cardiovascular illnesses which since has been proven to be a marker of illness best. Other folks with increased ranges of homocysteine are much more likely to have heart problems, however decreasing ranges doesn’t make center assaults and strokes much less more likely to happen. All those that rushed the science and bought nutritional dietary supplements to decrease their homocysteine had been flushing their cash down the drain.
Checking out the amyloid beta speculation
Although the impact of aducanumab, the Alzheimer’s drug evolved by means of biotechnology corporate Biogen, on laborious medical endpoints are lackluster, it’s been proven to cut back the formation of amyloid beta plaques in sufferers with early-stage Alzheimer’s. Amyloid beta denotes proteins that clump in combination to shape plaques often noticed in sufferers with Alzheimer’s. It’s been hypothesized that those plaques pressure the indicators and signs of Alzheimer’s. Animal fashions have proven that interfering with amyloid beta plaque formation may result in enhancements in functioning.
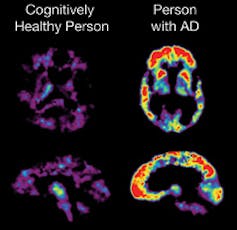
The Alzheimer’s Illness Training and Referral (ADEAR) Heart, NIH/Wikimedia Commons
The knowledge linking amyloid beta plaques to laborious medical endpoints isn’t a slam-dunk. Not like high blood pressure and increased LDL ldl cholesterol, which has been proved to be connected to cardiovascular occasions, amyloid beta has now not noticed such definitive effects.
Two massive medical trials assessing aducanumab were carried out, one who began with the next dose and one who began with a decrease dose that used to be later greater. Each trials had been stopped early, and the lower-dose trial discovered no advantages. The upper-dose trial discovered modest advantages in keeping up psychological functioning, however the trial didn’t have sufficient sufferers to turn that those advantages had been because of the drug and to not likelihood. After the reality, the researchers mixed knowledge from sufferers who gained high-dose aducanumab in each trials and located an development in psychological functioning. Alternatively, many mavens operating medical trials bristle at combining trial results like this: Those after-the-fact analyses were proven in some instances to now not pan out at some point.
Different to begin with promising experimental medicine concentrated on amyloid beta for Alzheimer’s additionally fell quick in lowering laborious medical endpoints of their medical trials. After this sort of medicine, solanezumab, failed to succeed in learn about objectives, further knowledge research post-trial instructed it could be efficient in a make a selection inhabitants with gentle Alzheimer’s. Researchers carried out an extra massive medical trial specializing in that subpopulation, however once more did not reveal important advantages. Nobody is aware of if aducanumab will in finding important advantages when the brand new medical trial completes or if it’s going to fail as solanezumab did.
If amyloid beta seems to be merely a marker and now not a reason behind Alzheimer’s, it’s going to be a pricey mistake: Aducanumab is estimated to price over US$56,000 a 12 months.
Used to be the FDA’s ruling a mistake?
Over 6 million American citizens now have Alzheimer’s illness, and deaths from Alzheimer’s have risen over 145% during the last two decades. Alzheimer’s illness now not best robs people in their autonomy but in addition puts an enormous burden on members of the family and the U.S. economic system: $355 billion is spent every year on taking good care of other folks with Alzheimer’s. Present FDA-approved therapies are best modestly efficient at controlling illness signs, and none goal a imaginable underlying reason.
The speeded up approval pathway lets in sufferers with early-stage Alzheimer’s to get admission to aducanumab whilst a bigger and extra definitive medical trial is carried out. Biogen says it hopes to have the medical trial finished by means of 2030. If the learn about does now not in finding discounts within the laborious medical endpoints, the drug will likely be withdrawn.

AP Picture/Steven Senne
If aducanumab is in the end discovered to be efficient, many sufferers with early-stage Alzheimer’s will reap the advantages in discounts in hospitalizations, physician visits, nursing house prices and societal burden.
If aducanumab is located to be useless, on the other hand, Medicare, insurers and sufferers may have spent tens of hundreds of thousands of bucks on a drug that now not best didn’t paintings but in addition uncovered sufferers to hostile occasions, together with the possibility of bleeding within the mind..
Will have to physicians prescribe aducanumab, and will have to insurers pay for it?
For sufferers within the previous phases of Alzheimer’s illness, there may be explanation why to take a look at aducanumab in line with the present medical trial knowledge and the loss of choices. However in improved illness, it’s not likely that aducanumab or any drug concentrated on amyloid beta will supply advantages.
In a cost-effectiveness evaluation of aducanumab, the Institute for Medical and Financial Evaluate, an impartial group assessing the price of scientific therapies, instructed an annual value vary from $8,300 to $23,000. This can be a a ways cry from the $56,000 a 12 months the corporate is anticipating to rate, and that doesn’t account for the hundreds of bucks in more checking out required to cut back the danger of mind swelling and bleeding.
The yearly charge of the drug will most likely a great deal exceed the fee financial savings in different spaces like lowered physician visits and hospitalizations. Till additional effects are launched, such excessive prices may lead non-public insurers not to quilt or rate upper copays for the drug. Given the typical age of the ones with Alzheimer’s illness, on the other hand, the general public receiving aducanumab will likely be eligible for Medicare and can perhaps be coated. Whether or not the drug will if truth be told deal with the illness – the largest factor in query – stays unsure.
Allow us to all hope that the FDA’s gamble will pay off.
Supply By means of https://theconversation.com/the-fdas-big-gamble-on-the-new-alzheimers-drug-162396